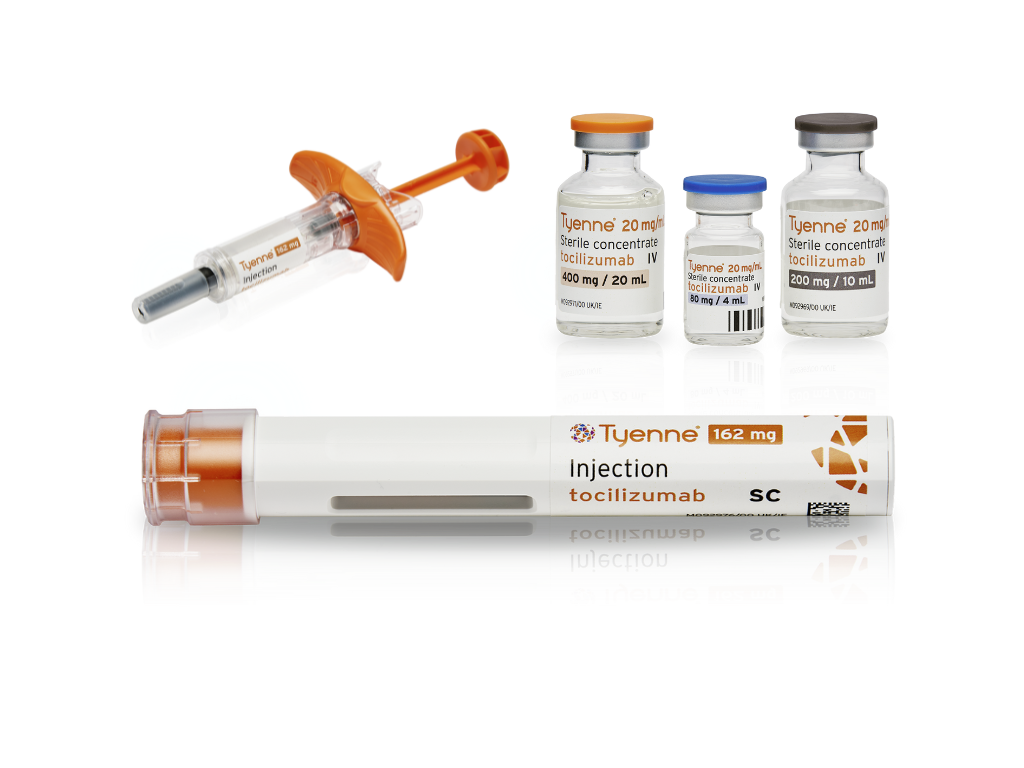
Tyenne 162mg solution for injection in a pre-filled syringe and pre-filled pen are indicated for use in the following conditions. Please consult the SmPC before prescribing. Tyenne treatment should be initiated by healthcare professionals and supervised by specialist physicians experienced in the diagnosis and treatment of conditions for which Tyenne is indicated.
- Tyenne, in combination with methotrexate (MTX), is indicated for the treatment of severe, active and progressive rheumatoid arthritis (RA) in adults not previously treated with MTX*
- Tyenne, in combination with methotrexate, is indicated for the treatment of moderate to severe active RA in adult patients who have either responded inadequately to, or who were intolerant to, previous therapy with one or more disease-modifying anti-rheumatic drugs (DMARDs) or tumour necrosis factor (TNF) antagonists*
- Tyenne pre-filled syringe only: is indicated for the treatment of active systemic juvenile idiopathic arthritis (sJIA) in patients 1 year of age and older, who have responded inadequately to previous therapy with NSAIDs and systemic corticosteroids. Tyenne can be given as monotherapy (in case of intolerance to MTX or where treatment with MTX is inappropriate) or in combination with MTX.
- Tyenne pre-filled pen only: is indicated for the treatment of active systemic juvenile idiopathic arthritis (sJIA) in patients 12 years of age and older, who have responded inadequately to previous therapy with NSAIDs and systemic corticosteroids. Tyenne can be given as monotherapy (in case of intolerance to MTX or where treatment with MTX is inappropriate) or in combination with MTX.
- Tyenne pre-filled syringe only: in combination with methotrexate (MTX) is indicated for the treatment of juvenile idiopathic polyarthritis (pJIA; rheumatoid factor positive or negative and extended oligoarthritis) in patients 2 years of age and older, who have responded inadequately to previous therapy with MTX.
- Tyenne pre-filled pen only: in combination with methotrexate (MTX) is indicated for the treatment of juvenile idiopathic polyarthritis (pJIA; rheumatoid factor positive or negative and extended oligoarthritis) in patients 12 years of age and older, who have responded inadequately to previous therapy with MTX.
- Tyenne is indicated for the treatment of Giant Cell Arteritis (GCA) in adult patients.
- Tyenne, in combination with methotrexate, is indicated for the treatment of severe, active and progressive rheumatoid arthritis (RA) in adults not previously treated with MTX.*
- Tyenne, in combination with methotrexate, is indicated for the treatment of moderate to severe active RA in adult patients who have either responded inadequately to, or who were intolerant to, previous therapy with one or more disease- modifying anti-rheumatic drugs (DMARDs) or tumour necrosis factor (TNF) antagonists.*
- Tyenne is indicated for the treatment of coronavirus disease 2019 (COVID-19) in adults who are receiving systemic corticosteroids and require supplemental oxygen or mechanical ventilation
- Tyenne is indicated for the treatment of active systemic juvenile idiopathic arthritis (sJIA) in patients 2 years of age and older, who have responded inadequately to previous therapy with NSAIDs and systemic corticosteroids. Tyenne can be given as monotherapy (in case of intolerance to MTX or where treatment with MTX is inappropriate) or in combination with MTX.
- Tyenne in combination with methotrexate (MTX) is indicated for the treatment of juvenile idiopathic polyarthritis (pJIA; rheumatoid factor positive or negative and extended oligoarthritis) in patients 2 years of age and older, who have responded inadequately to previous therapy with MTX. Tyenne can be given as monotherapy in case of intolerance to MTX or where continued treatment with MTX is inappropriate.
- Tyenne is indicated for the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS) in adults and paediatric patients 2 years of age and older.